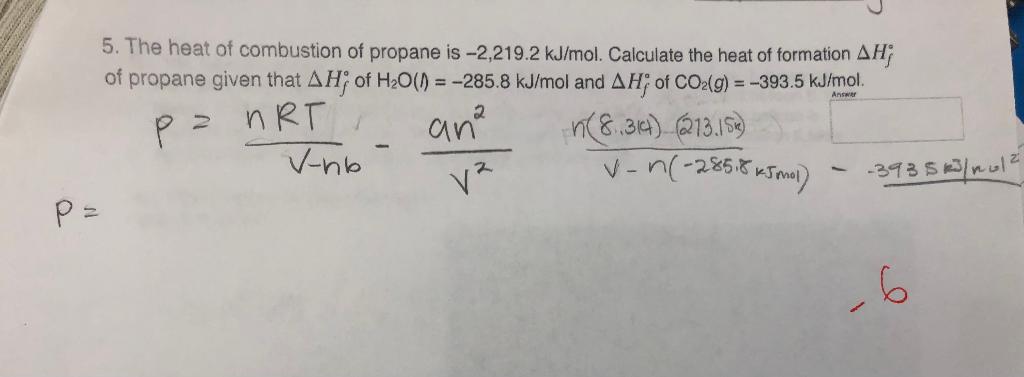Calculate the standard heat of formation of propane, if its heat of combustion is -2220.2 KJ mol^-1 , the heats of formation - Sarthaks eConnect | Largest Online Education Community

3. The standard heat of combustion of propane is -2220.1 kJ mol-! The standard heat of varonisation of liquid water is 44.0 kJ mol-. What is AH of - CoHs (9) +502 (
enthalpy of combusstion of propane, bu†an e, and pen†an e are 2220, 2878, 3537 kj/mole respectively . order of calorific value (per gram ) of fuel will b

SOLVED: Consider the combustion of propane: C3H8(g) + 5O2(g) â†' 3CO2(g) + 4H2O(g) ΔH = -2221 kJ Assume that all of the heat comes from the combustion of propane. Calculate ΔH when
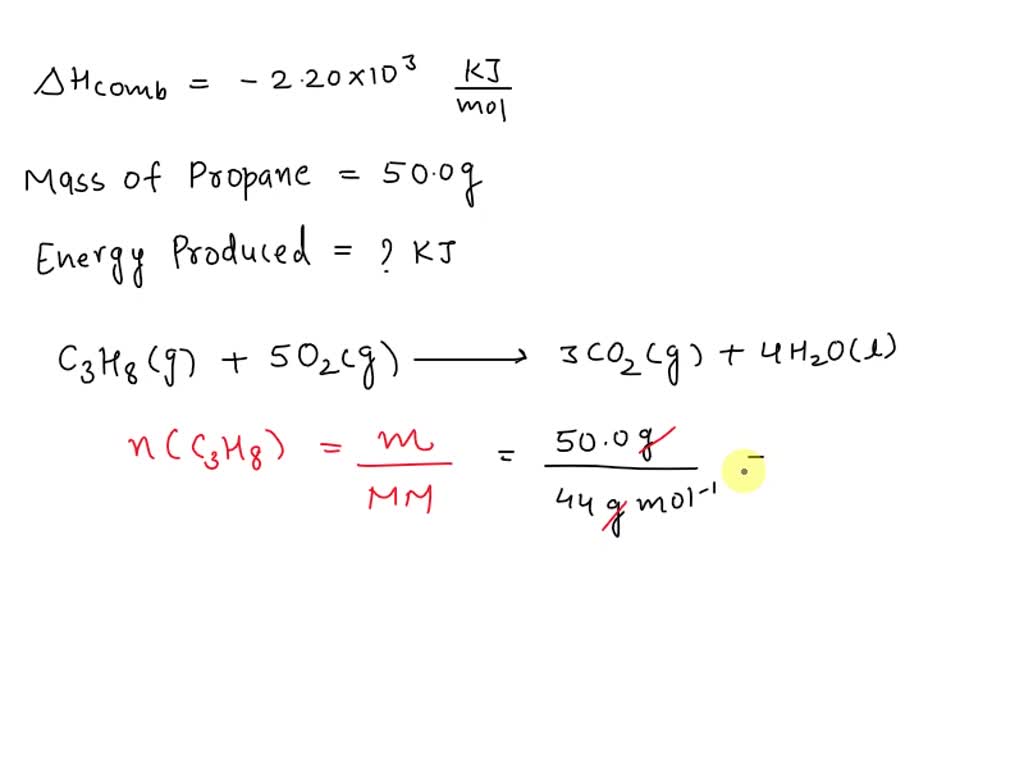
SOLVED: The heat of combustion of propane is -2.20E3 kJ/mol. Calculate the energy in kJ produced when 50.0 g of propane is burned in excess oxygen.
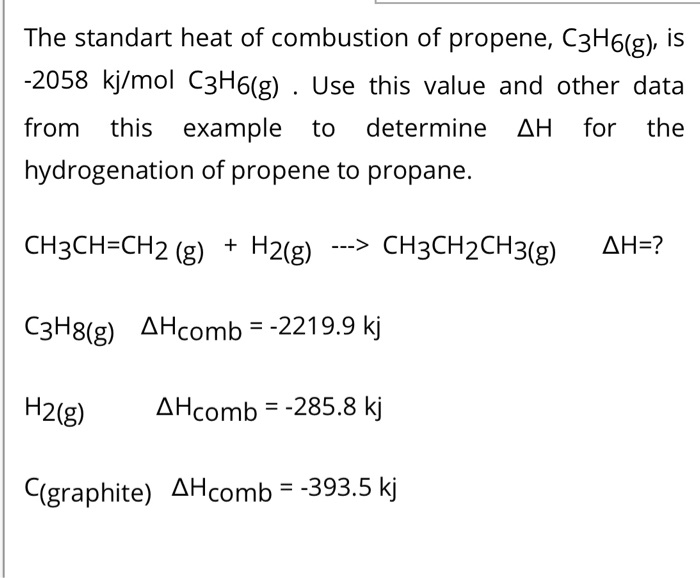
SOLVED: The standard heat of combustion of propene, C3H6(g), is -2058 kJ/mol C3H6(g). Use this value and other data from this example to determine ΔH for the hydrogenation of propene to propane:

SOLVED: Consider the combustion of propane, C3H8: C3H8(g) + 5 O2(g) â†' 3 CO2(g) + 4 H2O(g) ΔH = -2252 kJ All of the heat from the combustion of a sample of
Calculate the standard heat of formation of propane, if its heat of combustion is 0-2220.2 KJ mol^-1 the heats of formation - Sarthaks eConnect | Largest Online Education Community
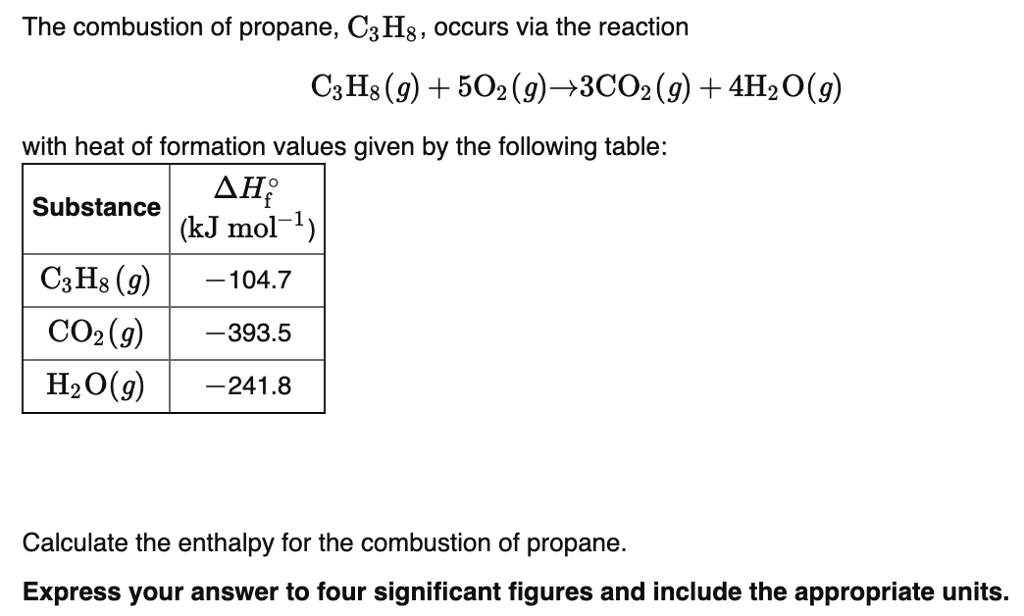
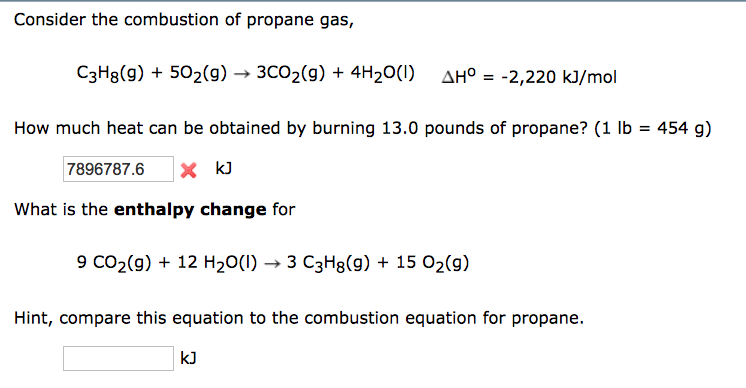
SOLVED: The combustion of propane, C3H8, occurs via the reaction C3H8(g) + 5O2(g) â†' 3CO2(g) + 4H2O(g) with heat of formation values given by the following table: Substance ΔHf (kJ mol-1) C3H8(g) -

3. The standard heat of combustion of propane is -2220.1 kJ mol-! The standard heat of varonisation of liquid water is 44.0 kJ mol-. What is AH of - CoHs (9) +502 (

The standard heat of combustion of propane is -2220.1 kJ/mol. The standard heat of vaporization of liquid water is 44 kJ/mol. What is the triangle H^{o} of the reaction :C_{3} H_{8} (g)+

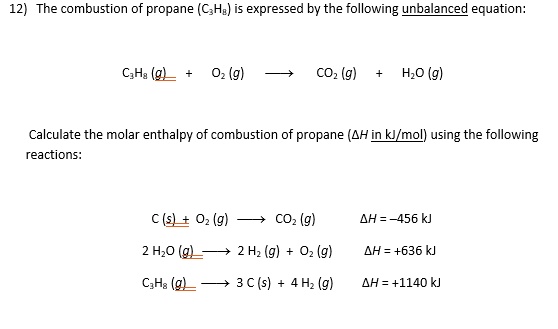
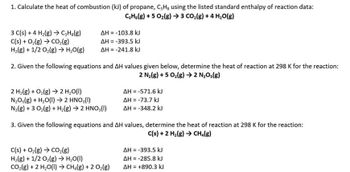
SOLVED: Calculate the heat of combustion (in kJ) of propane (C3H8) using the standard enthalpy of reaction data provided. C3H8(g) + 5 O2(g) –> 3 CO2(g) + 4 H2O(g) ΔH = ?
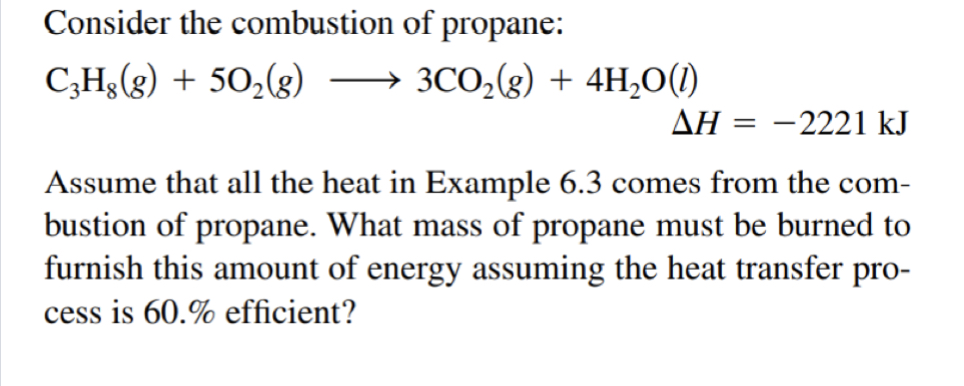
SOLVED: Consider the combustion of propane: C3H8(g)+5 O2(g) ⟶ 3 CO2(g)+4 H2O(l) Δ H=-2221 kJ Assume that all the heat in Example 6.3 comes from the combustion of propane. What mass of
![The HRR ramp of the propane burner in the experiments [11, 12] and the... | Download Scientific Diagram The HRR ramp of the propane burner in the experiments [11, 12] and the... | Download Scientific Diagram](https://www.researchgate.net/publication/329589405/figure/tbl1/AS:703057390231556@1544633352265/The-HRR-ramp-of-the-propane-burner-in-the-experiments-11-12-and-the-corresponding-mass.png)
The HRR ramp of the propane burner in the experiments [11, 12] and the... | Download Scientific Diagram

A 10.0 g sample of propane, C3H8, was combusted in a constant-volume bomb calorimeter. The total heat - Brainly.in